THC Oxidation to CBN
CBN is the oxidative degradation product of THC. It is more stable than THC due to its conjugated structure.
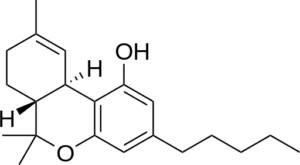
![]()
THC- Delta 9 CBN –New double bonds-Hydrogens lost here
The change is the aromatization of the top ring. 4 hydrogen atoms are lost, and two new double bonds are formed. Creating a double bond where there were hydrogens is an oxidation process, where as adding hydrogens to a double bond is a reductive process. Radical oxygen is responsible for the atmospheric degradation of THC to CBN. There are other chemical processes to synthesize CBN from THC as well.
THC Reduction to HHC

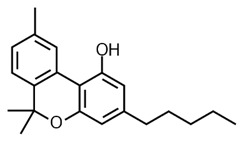
![]()
THC Delta 9 Hexahydrocannabinol (HHC)
Hexahydrocannabinol has a saturated (full hydrogen capacity, no double bonds) top ring. In this sense, it is the opposite of CBN. CBN is oxidized THC and HHC is reduced THC.
THC Delta 9 Isomerization to Delta 8 and Delta 10
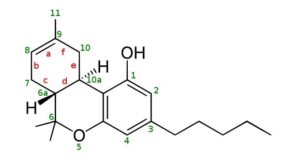
THC Delta 8, Double Bond is between 8 and 9
Delta-9, the most common and the natural form of THC, is actually the least stable.
Delta 8 has the double bond in a more thermodynamically stable position, and delta 10 even more so due to its conjugation with the bottom aromatic ring.
Permalink
Dear sir
We are used lube oil recycling victory in disttiltion process.
Our problem odder smells in production
We use belching clay.
Do you have any system to remove bad odder smells for oil.
Regards
Al
Saudi Arabia
Contact 00966 555450 148
Permalink
Hello,
One of our products may suit your needs. Please contact pvt@brinstrument.com as he will be able to best help you. Thank you!
Best,
Luke
Permalink
Informative.
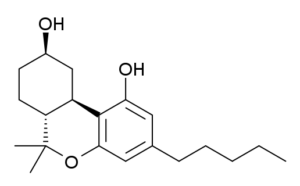
Only part that seems off is this “CBN is oxidized THC and HHC is reduced THC”
Is it still even Thc with a hydroxy group instead of a methyl group at the 11? ??
Permalink
Oops, that is definitely a different molecule than I meant to show. There should just be that methyl group on the top ring and not the hydroxy. Thanks!
Permalink
It would definitley be a diol of some sort..